Primary Research Interests/Projects
Our research has focused on the actions of two putative neuromodulators that we hypothesize may serve as endogenous suppressers of epileptic neuronal activity and epileptogenesis, the process by which the brain becomes epileptic. The first of these is arachidonic acid, which is a polyunsaturated fatty acid that is metabolized to bioactive lipid mediators upon its release from membrane phospholipid stores. Metabolism of free arachidonic acid can occur in the brain via several pathways. However, we are specifically interested in the cyclooxygenase (COX) pathway. This pathway is a key target of nonsteroidal anti-inflammatory drugs (NSAIDS), including aspirin and ibuprofen, which derive much of their therapeutic benefit through inhibition of COX catalytic activity. Although there are two COX isoforms, COX-1 and COX-2, the subject of our studies is the second inducible isoform because its expression and activity have been linked directly to increased excitatory neuronal activity associated with seizures. Initial results from studies employing transgenic and pharmacological approaches in animal models of epilepsy support the contention that COX-2 activity serves to suppress seizure activity acutely and antagonizes epileptogenesis chronically. Future studies will strive to elucidate the site of this activity within the neurocircuitry of the brain and identify the specific product(s) of COX-2 involved (e.g., prostaglandins). More details on the function of this signaling pathway in the biology and pathobiology of the CNS can be obtained from our review in Pharmacology and Therapeutics (2006).
A second related area of research in the laboratory explores the possible function of endogenous Interleukin-1β (IL-1β) in epilepsy. It is becoming clear that this classic immune cytokine has novel neuromodulatory actions within the CNS that are independent of its functions in immunity. Our results from studies in transgenic mice that lack endogenous IL-1β signaling have led us to conclude that, like COX-2, endogenous IL-1β functions to suppress seizure activity. Moreover, preliminary evidence suggests that its actions in the CNS may protect neurons from injury induced by status epilepticus, a condition of severe persistent seizure activity that is a risk factor for acquired epilepsy. Interestingly, evidence suggests that the neuromodulatory actions of IL-1β in the epileptic brain may require COX-2 activation, thus linking this research project with the COX-2 project described above. Future studies will continue to explore this relationship. Initial findings from this research are described in a manuscript that is in press in the journal, Neurobiology of Disease.
Ongoing collaborative research
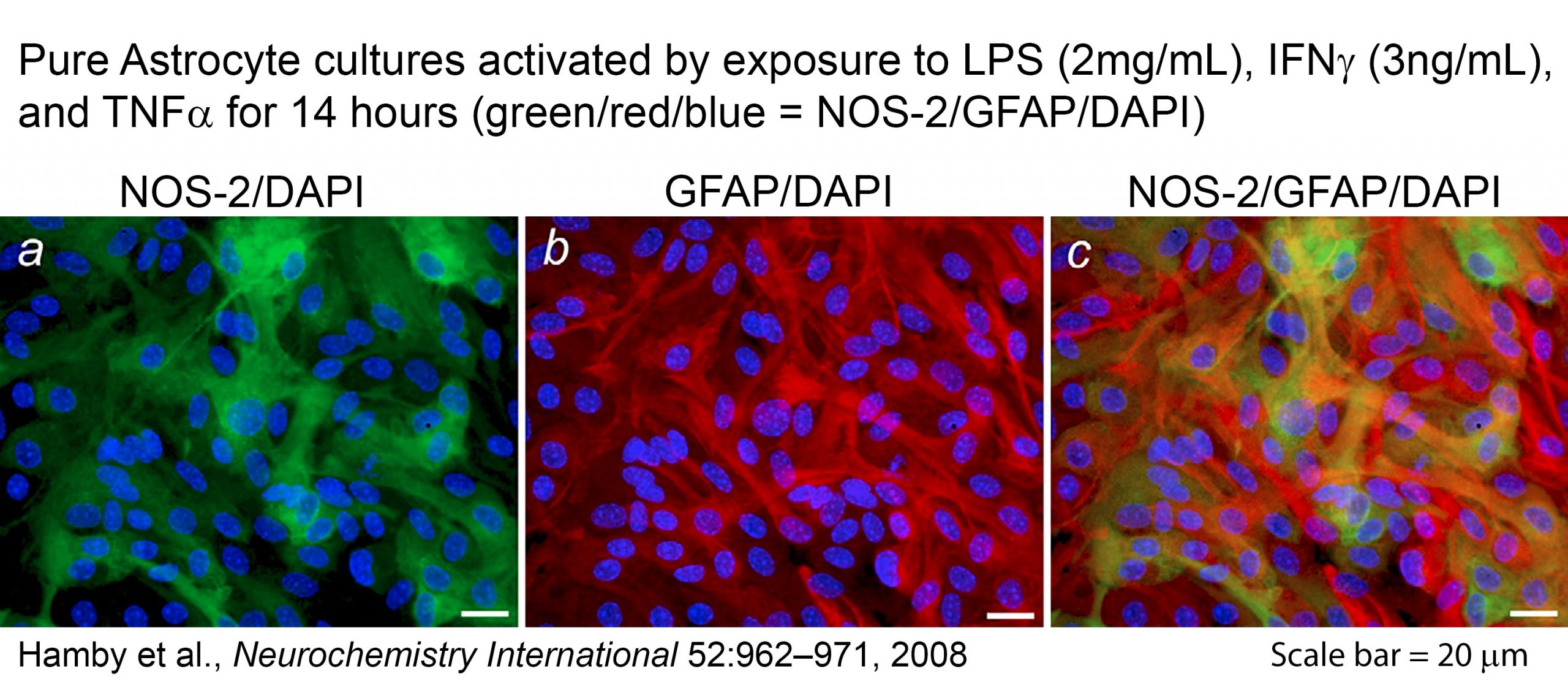
Additional research in collaboration with the laboratory of Sandra Hewett has centered on glial cell biology as it pertains to mechanisms of responsiveness within the astrocyte and microglia populations of the central nervous system. Results from this research have led us to propose the concept of population-based control of gene expression, whereby the intensity of the reaction of a population of cells to a given stimulus is determined to a large extent by the number of cells within the population that are recruited to respond. Our results suggest further that the number of cells that respond is determined not only by the nature of the stimulus but also by intrinsic heterogeneity within the cell population. Thesein vitro studies employ biochemical, immunofluorescence, and cell culture approaches to examine molecular aspects of gene regulation at the cellular and subcellular levels. Results from this ongoing project are described in research publications in the Journal of Neuroscience Methods (2006), Glia(2006 and 2010), Neurochemistry International (2008), and Prostaglandins and other Lipid Mediators(2008). This work also provided the impetus for a recent review article in the Journal of Neurochemistry(2009).